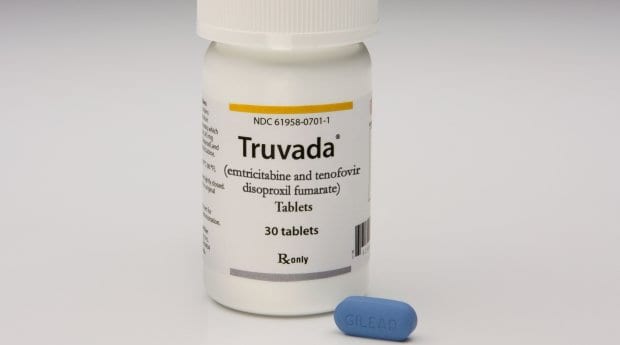
Getting access to Truvada could become easier for all Canadians in the near future.
The manufacturer of Truvada, a drug that has been shown to significantly reduce the risk of HIV infection, has filed an application with Health Canada to recognize the drug as an effective pre-exposure prophylaxis (PrEP).
In 2006, Health Canada approved Truvada as a treatment for HIV in combination with other drugs. It is not currently approved, however, for use as PrEP. Doctors can currently prescribe the pill “off-label” to patients, but that limits those who can access the drug — and without insurance, using Truvada as PrEP would cost roughly $900 a month.
Even though Health Canada has yet to give its seal of approval for Truvada as PrEP, clinical trials have produced significant results. A recent study conducted by Kaiser Permanente examined the efficacy of Truvada as PrEP among 600 men who have sex with men (MSM). After two and a half years, none of the participants had contracted HIV, despite the fact that half of them contracted another sexually transmitted infection during the study period.
A spokesperson for Gilead Sciences, which manufactures Truvada, said the filing to Health Canada was made in response to “interest from the clinical and HIV communities, with the aim of reducing the risk of sexually acquired HIV infection and the number of HIV infections.”
On April 28, 2015, Health Initiative for Men, a Vancouver-based gay men’s health organization, wrote an open letter calling on Gilead Sciences Canada to file an application with Health Canada.
“We think this is a positive step in terms of adding to the options for STI and HIV prevention among gay men,” says HIM’s executive director, Greg Oudman. “We were the authors of the open letter to Gilead urging them to seek approval and as we say in that letter we are hoping this process will move quickly and that Health Canada will act on Gilead’s request to approve Truvada as PrEP in Canada.”
The letter was endorsed by the AIDS Committee of Toronto, AIDS Vancouver, CATIE, YouthCO HIV & Hep C Society, Vancouver Coastal Health Regional HIV Program, REZO in Montreal, and the Canadian Association of Nurses in HIV/AIDS Care.
Now that the application has been filed, Health Canada scientists will review the drug to assess its safety, efficacy and quality for its use as PrEP.
“While it may not be possible to say precisely how long it will take to review Gilead’s submission, the department’s target review time for this type of submission, where a new indication is being sought, is 300 days,” a Health Canada spokesperson says.
HIM’s senior program manager, Jody Jollimore, says that HIM will continue promoting PrEP to men who can access the treatment.”
“I think we need to continue creating community awareness so people understand what PrEP is and if it’s for them and then work with individual candidates who come forward and say they want it. We will work to make it accessible. Some will pay out-of-pocket, some will have insurance and for others, unfortunately, it may mean a wait until there is a publicly funded program.”
Jollimore hopes that the growing body of evidence of Truvada’s efficacy as part of a comprehensive prevention strategy will expedite the approval process.
“It’s no longer something that people don’t know about,” he says. “The research is in. It’s an effective tool and even the doubters like AIDS Healthcare Foundation in the United States have come around and say that they believe PrEP is a prevention tool that should complement other prevention efforts.”
 Why you can trust Xtra
Why you can trust Xtra