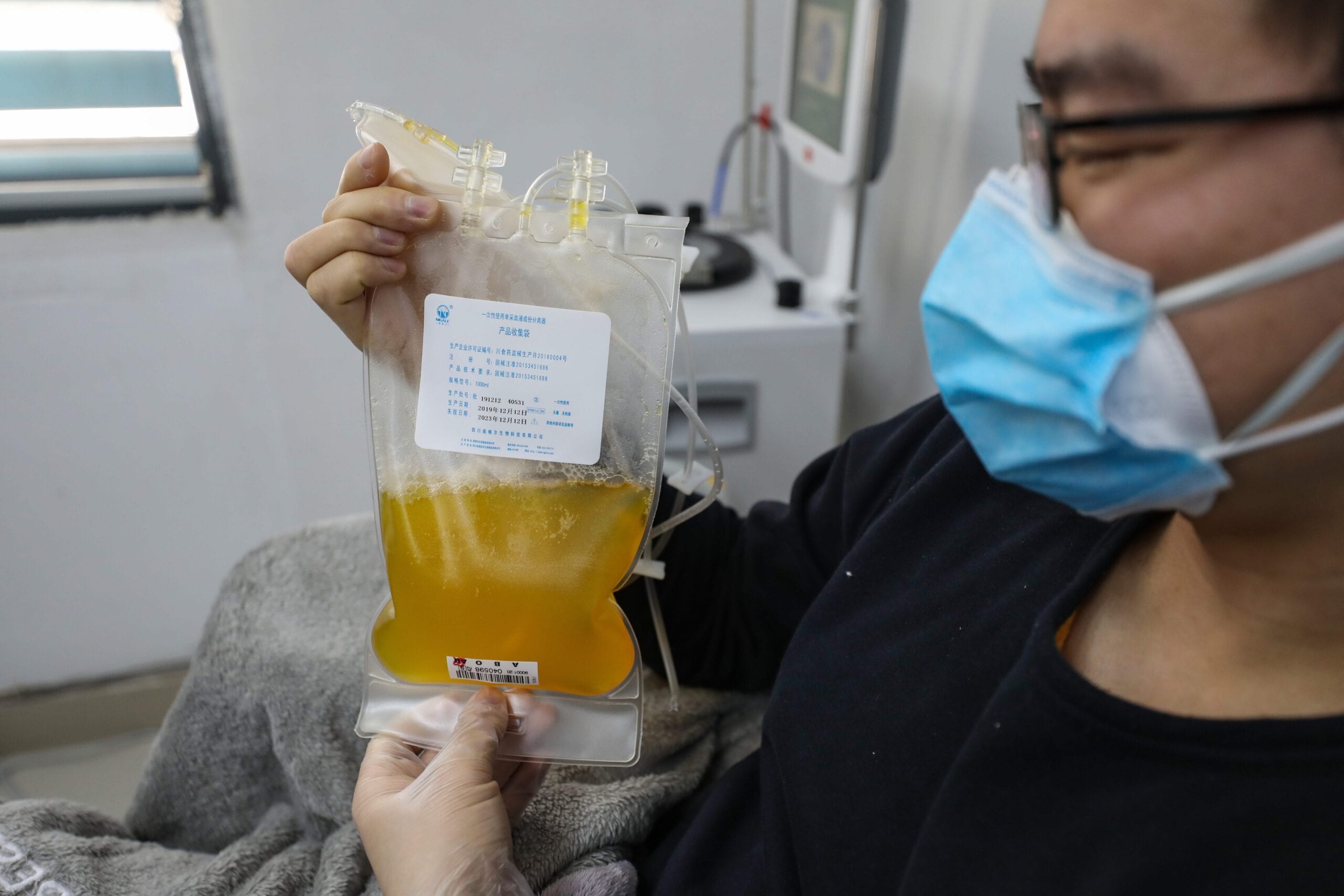
Despite the growing need across North America, queer men in Canada and the U.S. who have recovered from COVID-19 will be unable to donate blood plasma to help research efforts.
In Canada, the Convalescent Plasma for COVID-19 Research (CONCOR) trial will be investigating the possibility of using blood plasma from people who have recovered from COVID-19 to treat patients currently hospitalized with the virus. But because of the rules governing blood and plasma donations in Canada, men who have had sex with other men (MSM) within the past three months will not be allowed to donate. That ban also extends to trans women who have not undergone gender-affirming surgery and who have sex with men.
As of May 6, there have been 63,496 confirmed COVID-19 cases and 4,232 deaths in Canada, according to figures from the Canadian government. In the U.S., the Centers for Disease Control and Prevention report 1,193,813 confirmed cases and 70,802 deaths in the same time frame.
Cole Davidson, the press secretary to federal Minister of Health Patty Hajdu, did not comment on the donation restrictions for MSM and trans folks, instead referring questions to Health Canada, the federal body that regulates blood and plasma donation. And two of the lead researchers of the CONCOR trial—Dr. Donald Arnold, director of the McMaster Centre for Transfusion Research, and Dr. Jeannie Callum, a professor at the University of Toronto and transfusion medicine specialist at Toronto’s Sunnybrook Hospital—also referred questions about the exclusion of gay men to Health Canada or Canadian Blood Services (CBS) and its Quebec counterpart, Héma-Québec.
“Canadian Blood Services and Héma-Québec will be responsible for providing the donor plasma for use in this clinical trial and the plasma will be collected and processed according to the protocols already in place under their Health Canada authorizations,” writes Geoffroy Legault-Thivierge, a media relations officer at Health Canada, in an emailed statement. “[This] include[s] the current donor deferral for men who have had sex with another man in the past three months.”
Delphine Denis, the manager of media relations for CBS, says the organization will follow all current rules in providing plasma for the CONCOR trials. “We are proud to be part of CONCOR, a national clinical trial to test the safety and effectiveness of COVID-19 convalescent plasma as a possible treatment to help patients with the virus,” she writes in an email. “Canadian Blood Services’ role is to supply convalescent plasma to physicians caring for patients with the virus in the context of the clinical trial and under the authorization of Health Canada.
“At this time, we have not been asked to loosen any of Canada’s blood donor eligibility criteria as a result of the COVID-19 outbreak. While current evidence and risk modelling suggest COVID-19 is not transmissible through blood and blood products, we are tightening the criteria in response to the pandemic.”
In 2019, the exclusion period for MSMs in Canada was reduced to three months, down from one year. At the beginning of April, the Food and Drug Administration (FDA) in the U.S. announced a similar reduction due to the shortage of blood during the pandemic.
But critics of the exclusion policy say the ban is based on stereotypes of gay men and not on scientific evidence. They argue that Canada needs to move to a system based on the sexual behaviour of each potential individual donor. “We need a gender-neutral donor screening process based on scientific evidence,” says Nathan Lachowsky, the research director of the Vancouver gay men’s health organization Community-Based Research Centre. “We need better public policy and education campaigns to dispel stereotypes and fight misconceptions rather than contribute to them. This hurts our communities and our health.”
Many countries—including Italy, Spain and South Africa—have already switched to a behaviour-based system. And Lachowsky points out that others have found ways to work around any concerns about the safety of donations from gay men. “In France, MSMs are allowed to donate plasma intended for transfusion (not research), which is put under quarantine until donors return for testing; a similar system could be put in place for this research and in Canada.”
Gay men who are unable to donate blood or plasma say the exclusion is unfair and prejudicial, and an obstacle to health and scientific advances. Jack Turban, a resident physician in psychiatry at Massachusetts General Hospital, contracted the novel coronavirus in March. He has since recovered and returned to work, but is unable to donate plasma to research studies on the efficacy of convalescent plasma.
“It was a painful reminder that I live in a society that stigmatizes my identity,” Turban writes in an email. “Blanket policies that ban gay and bisexual men from donating blood and plasma are unscientific and discriminatory. They leave us with the message that we can only be ‘good’ or ‘pure’ if we are sexually abstinent, further perpetuating internalized homophobia, which is psychologically damaging.”
“Blood and plasma are both currently in short supply, and these policies make those shortages worse”
That health and government bodies would stick with exclusionary polices even during a crisis like the COVID-19 pandemic, shows the depth of the prejudice against gay men according to Turban. “It speaks to the fact that medical authorities are clouded by homophobic bias that leaves them unable to issue evidence-based guidance,” he adds. “Blood and convalescent plasma are both currently in short supply, and these policies make those shortages worse.”
But Turban says he has some hope that the realities of the pandemic may spark changes, at least in the U.S. “The FDA is currently saying that the new guidance will stay in place post-pandemic,” he says. “The FDA’s new guidance is a step forward, but it’s still discriminatory and unscientific. The ban should be lifted completely and we should switch to screening all donors using evidence-based risk factors: condom use, number of sexual partners and the use of pre-exposure prophylaxis which is known to reduce risk. I’m hopeful that the FDA will continue to reassess their policy and make progress.”
Denis says CBS is continuing to study the deferral period policy. “In Canada, any change to the blood and plasma donor eligibility criteria that could affect patients must be evidence based and approved by Health Canada, our regulator,” she says. “In partnership with Héma-Québec and with funding from Health Canada, we are supporting 15 research projects that will inform next steps for possible change to the criteria specific to men who have sex with men.”
But Turban says people in the U.S. and Canada should not wait for government bodies to take action. Instead, he says, now is the perfect time to apply pressure to politicians to enact changes to the existing policies. “I encourage people to contact their representatives to share their concerns about these unscientific and discriminatory policies,” he says.

 Why you can trust Xtra
Why you can trust Xtra