New guidelines from the US Centers for Disease Control and Prevention (CDC) recommend expanded use of PrEP (pre-exposure prophylaxis), advising medical providers to consider the daily HIV-prevention pill for uninfected, at-risk patients.
According to the guidelines issued May 14, PrEP is recommended for anyone in an “ongoing sexual relationship” with an HIV-positive partner; gay or bisexual men who have had sex without condoms or have been diagnosed with sexually transmitted infections in the last six months and are not in “mutually monogamous relationships” with partners who recently tested HIV-negative; heterosexual men or women who do not always use condoms when having sex with partners at risk for HIV, like injection drug users; and any person who is at risk of HIV infection through injection drug use.
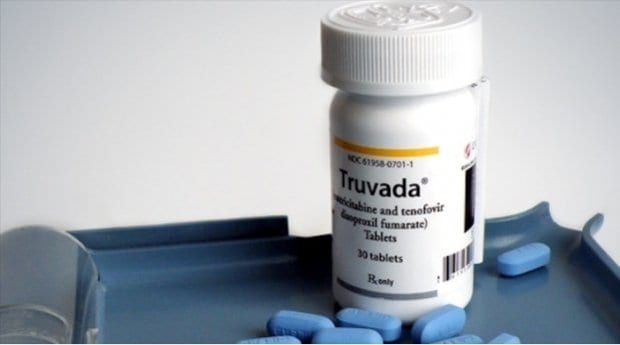
In 2012, the US Food and Drug Administration (FDA) approved the use of Truvada for use as PrEP in conjunction with other safer-sex tools, such as condom use and regular HIV testing.
“HIV infection is preventable, yet every year we see some 50,000 new HIV infections in the United States,” CDC director Tom Frieden says in a statement accompanying the new guidelines. “PrEP, used along with other prevention strategies, has the potential to help at-risk individuals protect themselves and reduce new HIV infections in the US.”
The guidelines underscore the need for HIV testing before PrEP is prescribed, as well as at three-month intervals while patients are on the drug regimen. “Regular testing ensures that anyone on PrEP who becomes infected with HIV discontinues PrEP use in order to minimize the risk that the virus could become resistant to the drugs,” the CDC says, adding that such patients can then start HIV treatment.
Advocates of the drug regimen welcome the new guidelines, saying that the CDC’s statement could motivate doctors, many of whom support it but don’t end up prescribing it, to recommend its use, The New York Times reports.
Michael Weinstein, president of the AIDS Healthcare Foundation, who dubbed Truvada a “party drug,” opposes the new guidelines, saying the CDC has “abandoned a science-driven, public health approach to disease prevention” in the US by recommending wider usage of PrEP when there is evidence that the drug regimen is often not followed as directed and there is “limited preventive effect at best,” The Bay Area Reporter says.
The CDC maintains that PrEP can reduce the risk of HIV infection by more than 90 percent when taken as advised, even as it acknowledges that inconsistent use results in much lower levels of protection.
Watch Xtra’s four-part video series on PrEP:
Part 1: Can a pill a day keep HIV away?
Part 2: A condom-free future?
Part 3: The controversy behind PrEP
Part 4: Why aren’t gay men taking PrEP?

 Why you can trust Xtra
Why you can trust Xtra