Gay men’s health and HIV prevention organizations are calling on the manufacturer of a drug that can significantly reduce the risk of HIV infection to file an application with Health Canada so that HIV-negative people in Canada can more easily access Truvada.
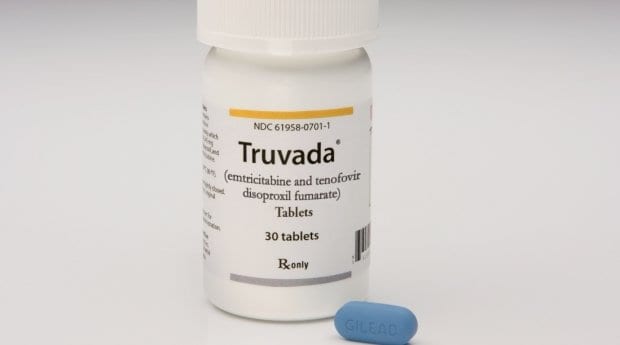
Manufactured by Gilead Sciences, Truvada can be used as a pre-exposure prophylaxis (PrEP) taken daily to prevent infection. Clinical trials show that taking the drug consistently can reduce HIV transmission by more than 86 percent.
It was approved for use as PrEP in the United States in 2012.
“We applaud Gilead’s support for PrEP in the United States and its recent announcement to seek approval in Australia but without formal approval in Canada it is not possible to establish publicly funded and regulated programs, or to evaluate cost effectiveness and safety,” reads an open letter authored by Vancouver’s Health Initiative for Men (HIM).
“This leaves gay men and other vulnerable groups with limited access to a proven prevention tool,” the letter states. “We find this policy inequitable and harmful to gay men’s health.”
The letter is endorsed by the AIDS Committee of Toronto, AIDS Vancouver, CATIE, YouthCO HIV & Hep C Society, Vancouver Coastal Health Regional HIV Program, REZO in Montreal, and the Canadian Association of Nurses in HIV/AIDS Care.
For Health Canada to consider approval for prescription medication, the drug manufacturer must submit an application.
A spokesperson for Gilead says the company has had “no recent communications with Health Canada regarding a PrEP filing.”
“We have submitted filings for Truvada for PrEP in Brazil, South Africa, Thailand and Australia, and at the request of the French National Agency for Medicines and Health Products Safety (ANSM), Gilead has provided data to permit an assessment of Truvada for a PrEP indication, which could result in a recommendation for temporary use in France,” the spokesperson says.
Without approval from Health Canada, doctors here can only prescribe the pill “off-label” for those who are willing and able to jump through “numerous technical hoops.”
“In order to obtain Truvada for PrEP, guys have to know what PrEP is, have a doctor who is willing to prescribe it, the money to pay for it, or health insurance to cover it,” explains HIM program manager Jody Jollimore. “We’re talking about a small fraction of guys.”
Without insurance the treatment can cost approximately $900 per month.
Jollimore notes that gay men and other men who have sex with men are the most at-risk population when it comes to HIV and have always been at the forefront of HIV prevention. “We use condoms, test and treat at higher rates than any other population,” he says.
“There is a new tool that gay men want,” he continues. “They are excited about it. They call our offices, ask our team, create Facebook groups — and those lucky enough to have a doctor to prescribe it, display it proudly on their sexual networking profiles.”
Jollimore says prevention agencies, health ministries and drug companies should be doing everything in their power to remove barriers between gay men and HIV preventions tools such as Truvada.
“I’ve been in rooms where questions have been asked about what the plans are for rolling out PrEP in Canada, and Gilead reps were in the room,” he says. “They know gay men in Canada are accessing Truvada off-label, even though they may not have had a letter presented to them saying so. We wanted to ask them in a very direct way to make application for this as they’ve done in other countries.”
Gilead Canada general manager Ed Gudaitis did not respond to Daily Xtra’s requests for comment by posting time.
Read the full letter to Gilead below and sign the petition here.
Ed Gudaitis, General Manager
Gilead Sciences Canada
6711 Mississauga Road, Suite 600
Mississauga, ON
L5N 2W3
April 28th, 2015
Dear Mr. Gudaitis:
On behalf of HIV prevention agencies, gay men’s health organizations and gay men in Canada, we are calling on Gilead Sciences to make immediate application to Health Canada for use of Truvada in HIV pre-exposure prophylaxis.
In December 2010 the New England Journal of Medicine published findings from the Gilead sponsored iPrEx trial examining the effectiveness of HIV pre-exposure prophylaxis (PrEP) in gay men and other men who have sex with men. The study ultimately showed that HIV was successfully prevented in over 90% of cases in men who were adherent to a daily dose of Truvada. Similar results were demonstrated in the subsequent PROUD trial from the UK and IPERGAY in France and Canada.
The advent of PrEP and its subsequent uptake by HIV-negative gay men, particularly in the United States, has been a welcome addition to the HIV prevention toolkit. Traditional interventions such as education, condoms, and treatment as prevention have been highly successful but they have failed to completely stem the tide of new infections. In 2011, the Public Health Agency of Canada estimated that the HIV incidence rate for men who have sex with men was 71 times higher than other men. In 2013 approximately 1000 Canadian gay men were diagnosed with HIV. These accounted for 50% of new infections in the country. Most become infected by partners who themselves are newly infected and/or unaware of their sero-positive status. PrEP offers additional protection to HIV-negative men who may find themselves in these situations.
In July 2012 the FDA approved the use of Truvada for PrEP in the United States and the US Centre for Disease Control developed clinical practice guidelines for administration of PrEP in vulnerable populations. Yet access to Truvada for PrEP in Canada remains elusive. Currently, it is prescribed “off-label” and treatment is administered under a patchwork of monitoring with little or no regulation. We applaud Gilead’s support for PrEP in the United States and its recent announcement to seek approval in Australia but without formal approval in Canada it is not possible to establish publicly funded and regulated programs, or to evaluate cost effectiveness and safety. This leaves gay men and other vulnerable groups with limited access to a proven prevention tool. We find this policy inequitable and harmful to gay men’s health.
At Health Initiative for Men, we consider PrEP to be a safe and effective HIV prevention tool when it is prescribed and administered in a regulated environment. We believe that barriers to access should be minimized for gay men and other vulnerable populations. A formally approved indication for the use of Truvada in HIV pre-exposure prophylaxis is the first step to achieving this goal. We further intend to actively petition public health officials at all levels for comprehensive PrEP guidelines and for approval of publicly funded programs. If we are to be serious about stopping HIV in all populations, and particularly in gay men, we must make PrEP more easily accessible. To quote from Chris Beyrer at the Center for Public Health and Human Rights in a recent Lancet publication, ‘Pre-exposure prophylaxis works – it’s time to deliver’.
Board of Directors,
Health Initiative for Men
Signatories:
AIDS Committee of Toronto
AIDS Vancouver
CATIE
REZO in Montreal
YouthCO HIV & Hep C Society
Vancouver Coastal Health Regional HIV Program
Canadian Association of Nurses in HIV/AIDS Care (CANAC)
Watch Daily Xtra’s video series on PrEP here.
 Why you can trust Xtra
Why you can trust Xtra