For years, researchers have been working to develop an alternative to oral HIV prevention—one that safely prevents infection without the burden of remembering to take daily pills. Now, it looks like an answer is finally here.
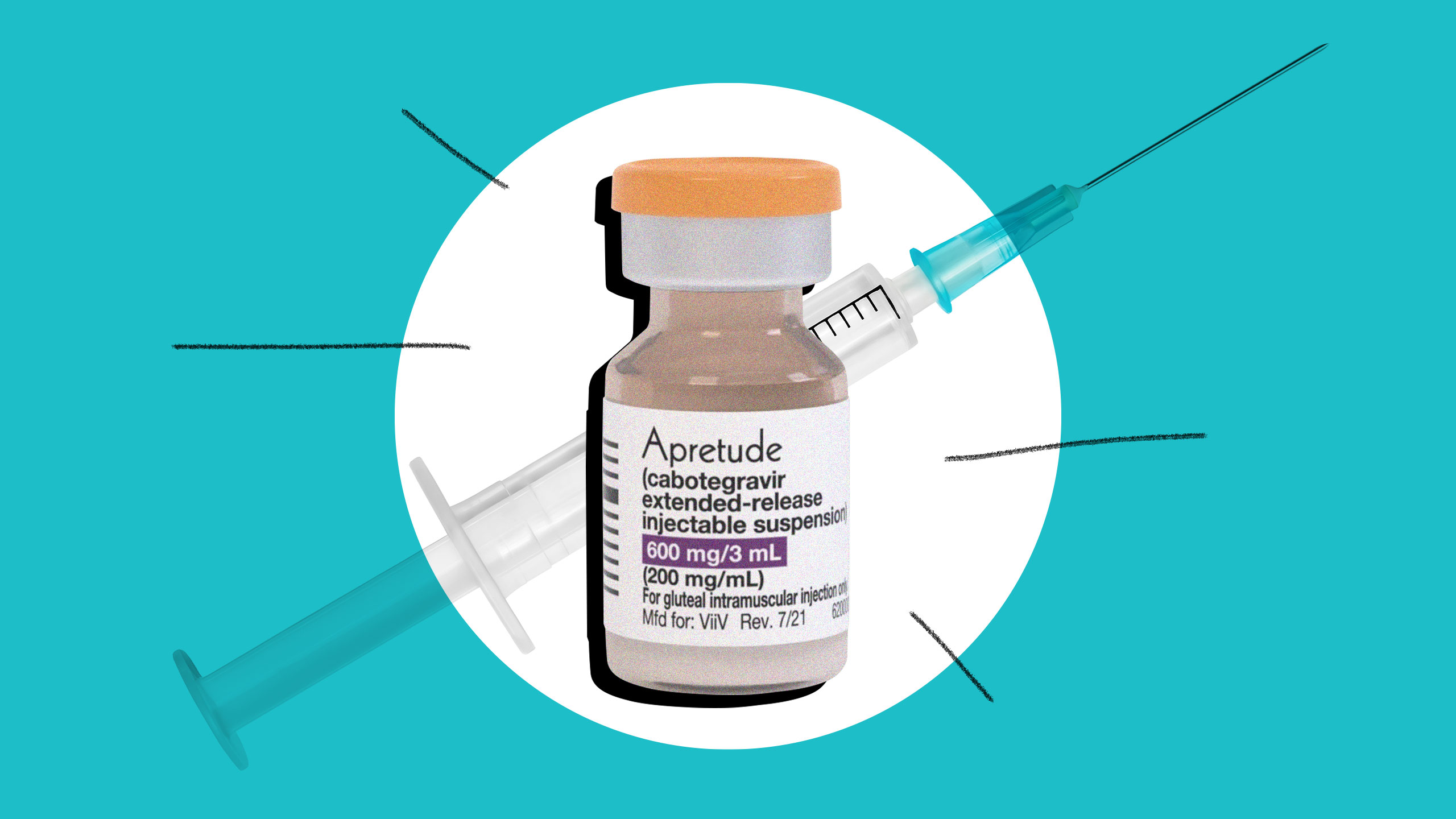
This week, the FDA approved Apretude, an injectable version of PrEP, for use in the U.S. The new treatment reportedly offers even more protection from HIV infection than daily pills. And the best part? It only needs to be taken every two months.
“This injection, given every two months, will be critical to addressing the HIV epidemic in the U.S., including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option,” Debra Birnkrant, director of the Division of Antivirals in the FDA’s Center for Drug Evaluation and Research said in a statement Monday.
Here’s what you need to know about this groundbreaking advancement.
What is PreP?
Pre-exposure prophylaxis, a.k.a. PrEP, refers to a medication people take to prevent contracting HIV during sex or injection drug use. Until now, PrEP has involved ingesting two pills, Truvada and Descovy, both of which are taken daily.
The U.S. Centers for Disease Control and Prevention say PrEP could be useful to anyone who’s had anal or vaginal sex in the past six months with a sexual partner who is HIV-positive, has not consistently used a condom or has been diagnosed with a sexually transmitted infection within the past six months.
That includes many men who have sex with men, trans women and cis women who are at risk of contracting HIV through vaginal sex.
What is Apretude?
Apretude was developed by ViiV Healthcare, which specializes in HIV research.
The FDA approval came on the heels of two large clinical trials: one that followed 4,566 cisgender men and transgender women who have sex with men, and another involving 3,224 cisgender women. Both trials found that Apretude was more likely to reduce HIV infection than the oral medications—by 69 percent for the first group and by 90 percent for the second.
That increase in effectiveness is largely due to how much easier it is for people to adhere to the injection schedule compared to remembering to take a daily pill.
“People who are vulnerable to acquiring HIV, especially those in Black and Latinx communities who are disproportionately impacted in the U.S., may want options beyond daily oral pills,” ViiV Healthcare CEO Deborah Waterhouse said in a statement. “With Apretude, people can reduce the risk of acquiring HIV with as few as six injections a year.”
Patients take two Apretude injections a month apart to start, before continuing on a once-every-two-months regime.
Apretude will be made available in the U.S. in early 2022.

 Why you can trust Xtra
Why you can trust Xtra