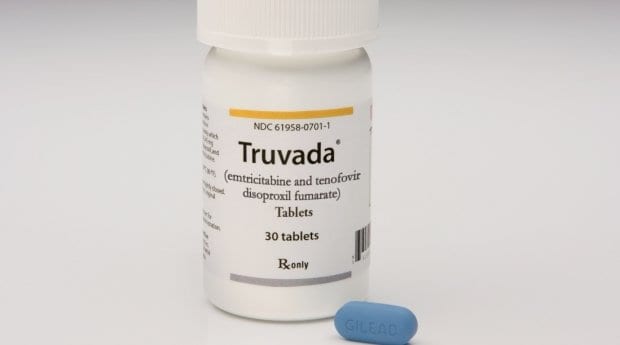
Health Canada has quietly approved Truvada for use as pre-exposure prophylaxis (PrEP) in Canada, according to documents in the government agency’s drug product database. Health Canada has not yet announced the change, but the drug’s product monograph is marked as updated Feb 23, 2016.
A new section now reads, “TRUVADA is indicated in combination with safer sex practices for PrEP to reduce the risk of sexually acquired HIV-1 infection in adults at high risk.” Health Canada did not initially respond to an emailed request for comment from Daily Xtra before publication, but later confirmed that Truvada has been authorized as preventive drug via an emailed response.
The statement says in part that “the drug is intended for use by high risk individuals — such as those whose sexual partner is HIV positive — in combination with safer sex practises, including condom use.”
Prior to this Truvada was already approved to treat HIV in Canada, but until now did not have the green light for use as HIV prevention. Studies worldwide have shown Truvada is safe, and as effective as condoms for preventing HIV infection. In the nearly four years since Truvada was approved as PrEP in the United States, researchers have only recently documented one case of a man on daily Truvada contracting HIV.
Even with Health Canada approval, however, Canadians may still struggle to access Truvada. Except for in Quebec — where the drug is mostly covered by provincial pharmacare — a monthly dose can cost over $900, and insurers have proved reluctant to give coverage. Some men have resorted to bringing generic drugs across the border from the United States to cut costs.
Joshua Edward, of Vancouver’s Health Initiative for Men, applauds the decision to approve PrEP, but says access is still the most important issue.
“The Health Canada approval means nothing, in terms of access to the general population,” he says. “I think it’s a fair question to ask why the province is paying for HIV medications that cost so much more and not willing to invest in supporting a preventative medication.”
Editor’s note: This story has been updated to include an emailed official confirmation from Health Canada that it has approved Truvada as PrEP.

 Why you can trust Xtra
Why you can trust Xtra